

11 Conversely, the prevalence of the VKORC1 allele that is associated with greatest increase in warfarin sensitivity is the highest in individuals of Asian descent. 6 8–10 The CYP2C9 alleles that are the most associated with increased warfarin sensitivity are more common in individuals of European descent compared with African or Asian descent. 3 6–9 Genetic factors influencing the response to VKAs include polymorphisms in the vitamin K epoxide reductase ( VKORC1) and cytochrome P450 2C9 ( CYP2C9) genes. The individual response to VKAs, such as warfarin, is influenced by demographics, clinical characteristics and genetic factors. As a class, compared with VKAs, these agents are at least as efficacious, are associated with a lower risk of major bleeding, have more predictable pharmacodynamics and require less frequent laboratory monitoring in individuals with VTE. 4 To overcome some of the limitations of VKAs, direct oral anticoagulants such as edoxaban, a potent factor Xa inhibitor, were developed. 3 Possibly due to this variability and the narrow therapeutic window of VKAs, their use is often associated with serious bleeding complications. 2 However, there is a high degree of interindividual variability in the dose–response relationship to VKAs, which necessitates frequent inconvenient laboratory monitoring of coagulation status. 1 Vitamin K antagonists (VKAs) with initial heparinisation have long been considered a mainstay for the management of VTE. Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant health problem with an estimated annual incidence of approximately 1– people among the general population. Adverse event (excluding bleeding) and discontinuation rates were similar among the three treatment groups. Warfarin therapy was proactively monitored throughout the trial, resulting in a median time within therapeutic range (TTR) of 68.4%. Complete information on the primary endpoint was ascertained for 99.5% of the total 56,346 patient-years of potential follow-up with only one patient lost to follow-up.
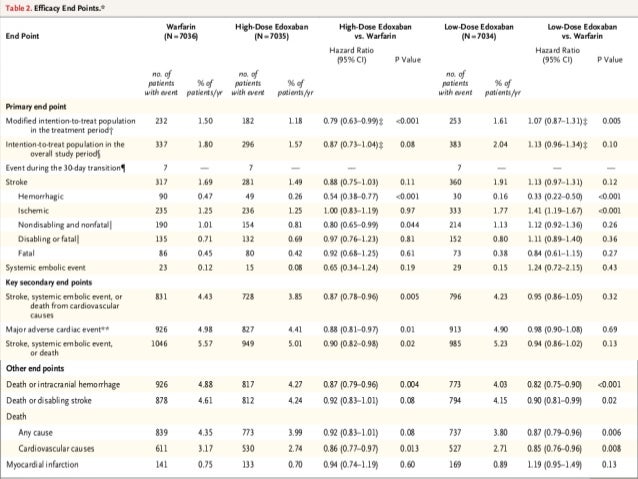
The principal safety outcome was adjudicated major bleeding. The primary efficacy outcome was the time to first adjudicated stroke or SEE. Edoxaban dosage was reduced by half if any of the following was present at randomization or during the course of the study: creatinine clearance 30-50 mL/min, bodyweight < or = 60 kg or concomitant verapamil, quinidine or dronedarone (standard dosing was resumed if the concomitant drug was discontinued and no other dose reduction factors were present). Patients were randomized (1:1:1) to receive warfarin (n = 7,036), edoxaban 60 mg (n = 7,035) or edoxaban 30 mg (n = 7,034). In addition, we specifically designed a comprehensive transition plan to protect patients from the undue risk of stroke and bleeding when switching to open-label anticoagulation at the end of the trial."
#Engage af timi 48 trial#
"In conducting this landmark trial, we sought to provide clinicians with robust data, evident by the trial size and follow-up, high percentage of time in therapeutic range for the warfarin treatment arm and very low rate of missing data. In addition, we identified an appropriate dose regimen for patients with clinical factors such as renal impairment and low body weight," said Robert Giugliano, M.D., S.M., FAHA, FACC, senior investigator, TIMI Study Group, physician cardiovascular medicine, Brigham and Women's Hospital, associate professor of medicine, Harvard Medical School, and co-global lead investigator, ENGAGE AF-TIMI 48 trial. "The results from the ENGAGE AF-TIMI 48 trial showed that edoxaban may provide a new treatment option for the prevention of stroke or systemic embolic events that demonstrates comparable efficacy to warfarin, while significantly reducing the risk of major bleeding.


 0 kommentar(er)
0 kommentar(er)
